Antifertility and Anti-Implantation Effects of Croton tiglium Seed Extracts in Female Albino Rats
| Received 02 May, 2025 |
Accepted 14 Sep, 2025 |
Published 30 Sep, 2025 |
Background and Objective: Croton tiglium, a Euphorbiaceae plant, has been traditionally used in African, Ayurvedic, and Chinese medicine for its purgative and contraceptive properties. Its seeds hold ethnopharmacological significance in fertility regulation practices. This study evaluated the antifertility and anti-implantation effects of Croton tiglium seed extracts in mature female albino rats. Materials and Methods: This study was conducted at the Animal Physiology Laboratory, Sudan University for Science and Technology, over three months. Thirty healthy female albino rats (150-180 g, 10-12 weeks) were randomly divided into five groups (n = 6). Ethanol and petroleum ether seed extracts of Croton tiglium were prepared using Soxhlet extraction and administered orally for 7 days post-mating. Mating was confirmed by sperm-positive vaginal smears, marking day 1 of pregnancy. On day 10, laparotomy was performed to assess implantation sites. Reproductive tissues were collected and processed for histopathology using H&E staining. The histologist was blinded to group allocation. Data were analyzed using ANOVA with significance at p<0.05. Results: The ethanol extract completely inhibited implantation, resulting in no pregnancies and high mortality, while the petroleum ether extract exhibited partial antifertility effects. The ethanol extract of Croton tiglium exerts potent antifertility and anti-implantation effects, primarily through direct uterine toxicity and disruption of endometrial histology. While ovarian tissue remains unaffected, the extract causes marked alterations in uterine and vaginal tissues, suggesting a localized site of action. In contrast, the petroleum ether extract shows a milder and reversible antifertility effect, implying a compound-specific mechanism. Histopathological analysis revealed significant uterine and vaginal changes in the ethanol-treated group, with no alterations in the cervix, fallopian tubes, or ovaries. Conclusion: These findings support the traditional use of Croton tiglium for contraception and suggest that its antifertility effect primarily operates via interference with uterine implantation.
| Copyright © 2025 EL-Kamali et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Croton tiglium, belonging to the Euphorbiaceae family, is well known in traditional medicinal practices, including Ayurveda and Traditional Chinese Medicine, for its strong purgative and contraceptive effects1,2.
The seeds of C. tiglium have historically been used in various cultures as a part of herbal formulations for family planning and fertility regulation. The plant contains several bioactive compounds, including phorbol esters, flavonoids, and diterpenoids, which are believed to contribute to its pharmacological effects3,4.
Despite its longstanding ethnomedicinal use, there remains a lack of comprehensive scientific studies evaluating the reproductive toxicity and antifertility potential of C. tiglium, particularly concerning its effects on implantation and ovarian function. Given the growing interest in plant-based contraceptives, it is crucial to assess the safety and efficacy of such traditional remedies using controlled laboratory models5.
This study aims to evaluate the antifertility and anti-implantation effects of ethanol and petroleum ether extracts of Croton tiglium seeds in female albino rats. Additionally, the study includes histopathological analysis of reproductive tissues to better understand the underlying mechanisms.
MATERIALS AND METHODS
Study area: This study was conducted in the Department of Physiology, Sudan University for Science and Technology, located in Khartoum, Sudan, and was carried out in September, 2022. The laboratory has standard animal research facilities, including housing, breeding, and surgical procedures. All experimental procedures were performed under institutional ethical guidelines for the care and use of laboratory animals, ensuring appropriate environmental conditions such as controlled temperature (22±2°C), a 12 hrs light/dark cycle, and unrestricted access to food and water.
Animals: Eighteen healthy, mature female albino rats (Rattus norvegicus) used in this study were obtained from the Animal House of the Faculty of Veterinary Medicine, University of Khartoum, Sudan. The animals were acclimatized for one week under standard laboratory conditions before the commencement of the experiment, weighing between 150-180 g, were housed under standard laboratory conditions (12 hrs light/dark cycle, 22±2°C, with free access to food and water). The animals were acclimatized for one week before experimentation.
Ethical approval: Ethical approval was obtained in accordance with institutional guidelines for the care and use of laboratory animals.
Grouping and treatment: The rats were randomly divided into three groups (n = 6 per group):
| • | Group I (control): Received distilled water orally | |
| • | Group II (ethanol extract): Received ethanol seed extract of Croton tiglium at 200 mg/kg b.wt., orally | |
| • | Group III (petroleum ether extract): Received petroleum ether seed extract of Croton tiglium at 200 mg/kg b.wt., orally |
Treatments were administered once daily for 7 consecutive days. Following treatment, each female was housed overnight with a proven fertile male at a 1:1 ratio. The presence of a vaginal plug the next morning was considered evidence of successful mating (day 1 of gestation)5.
Assessments: After a 14-day mating period, all animals were sacrificed on day 15 of gestation. The following assessments were conducted:
| • | Pregnancy outcomes: Number of pregnant rats per group | |
| • | Implantation site counts: Uteri were examined for visible implantation sites6 | |
| • | Histological examination: Reproductive organs (uterus, cervix, vagina, fallopian tubes, and ovaries) were dissected and preserved in 10% formalin for histopathological analysis using hematoxylin and eosin staining under light microscopy, an Olympus CX23 binocular microscope (Olympus Corporation, Tokyo, Japan) for histological examination of stained tissue sections |
Statistical analysis: Data obtained from the experimental groups were statistically evaluated as Mean±SD7 for each group.
RESULTS AND DISCUSSION
Pregnancy and implantation outcomes:
| • | Control group: All rats conceived and delivered 12 L; average implantation sites = 12.5±0.22 | |
| • | Ethanol group: No pregnancies; implantation sites = 0; 4 of 6 rats died, indicating high toxicity and complete antifertility | |
| • | Petroleum ether group: Partial effect; 4 rats delivered 5 L; average implantation sites = 5.0±0.31 |
Histopathological findings
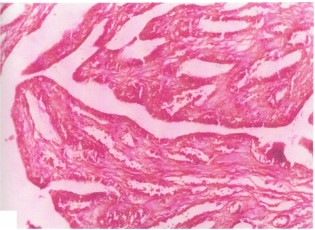
Uterus: Histopathological examination of animal’s uterus after the end of the experiment in which females animals were mated with males after treatment with ethanol seeds extract or petroleum ether seeds extract revealed that in their uterine tissues, the lining epithelial cells of endometrium and gland were hyperamic as shown in Fig. 1 (control uterus), Fig. 2 and 3 when compared with control uterus (Fig. 1.) Glands at stratum baseless shows necrosis and shedding of epithelial cells and some glands were dilated and lined by flatten epithelial cells as can be seen in Fig. 4. Erosion of superficial layers associated with odema and infiltration of mono nuclear cells as seen in Fig. 5.
|
|
|
|
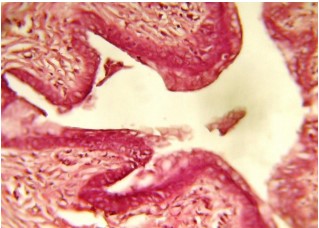
Figure 1 shows a cross-section of the uterus from the control group of rats normal uterine structure), stained using Hematoxylin and Eosin (H&E) stain at 40x magnification. The histological image demonstrates the typical structure of a healthy uterus, with well-preserved layers and tissue organization. The uterine tissue in this section would show the distinct layers that make up the uterus, including: (1) Endometrium (inner lining of the uterus): This layer should appear well-defined with a normal glandular structure. (2) Myometrium (muscle layer): This layer should appear intact and evenly distributed, without signs of inflammation or structural damage. (3) Serosa (outer layer): The outer covering of the uterus should show no signs of fibrosis, adhesions, or other abnormalities.
The tissue structure in a normal uterus would show a high degree of organization, with epithelial cells lining the endometrium arranged in a single layer or a few layers, depending on the stage of the estrous cycle.
In the context of this experiment, Fig. 1 represents the control group (group I), which was given distilled water and did not receive any treatment with Croton tiglium extracts. This serves as the baseline for comparison against the experimental groups treated with Croton tiglium extracts (ethanol or petroleum ether8-10.
|
The histological images supported the physiological findings: In rats treated with the ethanol extract, the uterine tissues showed: (i) Hyperplasia and hyperemia (increased blood flow). (ii) Necrosis, glandular damage, and erosion of the uterine lining. (iii) These pathological changes render the uterus non-receptive to embryo implantation.
A transverse section of the uterus from rats administered 200 mg/kg b.wt., of Croton tiglium ethanol extract. Hematoxylin and Eosin (H&E), 40× magnification.
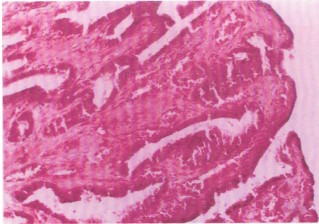
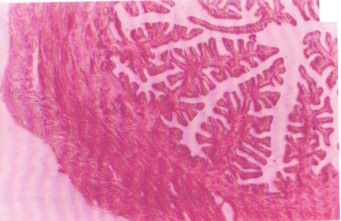
The endometrial glands appear markedly atrophic, characterized by reduced gland size and diminished epithelial activity. These changes indicate functional impairment, likely due to cytotoxic effects of the extract, and may underlie the observed anti-implantation outcomes. Uterine glands are crucial for producing secretions that support embryo implantation. Atrophy reflects compromised secretory function, possibly due to epithelial degeneration or necrosis (Fig. 2). Compared to the control (Fig. 1), which shows normal gland morphology, these alterations highlight the direct uterine toxicity of the ethanol extract and support its antifertility potential.
Section of uterus from treated rats (200 mg/kg Croton tiglium ethanol extract). The H&E stain, 80× magnification (Fig. 3). Presence of hyperplastic endometrial cells, indicating excessive cellular proliferation. Hyperaemia, evidenced by engorged blood vessels, suggests a local inflammatory response.
Hyperplasia may result from cellular stress or compensatory regeneration following injury. The associated vascular congestion supports an inflammatory or irritant-mediated tissue reaction, further disrupting uterine receptivity and implantation.
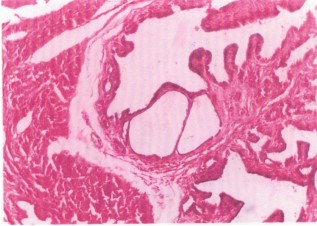
Uterine section at high magnification (H&E, 300×) post ethanol extract administration (Fig. 4). Dilated yet atrophic glands with flattened epithelial cells. Epithelial detachment at the stratum basalis, indicating basement membrane disruption. Cross-section of uterus from treated rats. H&E stain, 80×. Erosion of the luminal epithelium (Fig. 5). Oedema and cellular infiltration indicative of acute inflammation. Epithelial erosion compromises the first barrier for embryo attachment. Oedema and immune cell infiltration further disturb the microenvironment, rendering the uterus non-receptive.
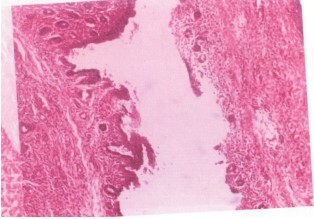
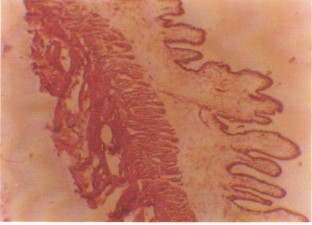
Vagina: Both extracts induced epithelial hyperplasia, vascular dilation, and lymphocyte infiltration, suggesting estrogenic or inflammatory effects potentially impacting sperm viability or mucosal defense (Fig. 6).
|
Vaginal histopathology
Findings in treated rats:
| • | Columnar epithelial hyperplasia | |
| • | Vascular dilation | |
| • | Lymphocytic infiltration |
These changes may reflect estrogen-like or irritant activity, which could impair sperm survival or motility, further contributing to infertility. Consistent with hormonal imbalance, possibly via estrogenic stimulation or anti-progesterone action.
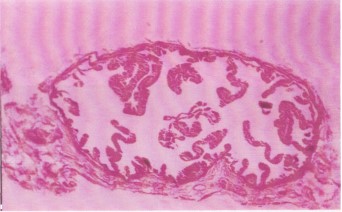
Cervix, fallopian tubes, and ovaries: No histopathological changes were observed, indicating that antifertility effects were localized primarily to the uterus and vagina (Fig. 7-9).
Ovarian tissue from treated rats exhibits normal architecture, with no apparent morphological damage at 40× magnification. The absence of ovarian pathology suggests that the antifertility effects are localized to the uterus and vagina, rather than due to systemic hormonal suppression or gonadal toxicity.
The ethanol extract of Croton tiglium demonstrated a potent antifertility effect via complete inhibition of implantation and marked uterine pathology. Histological damage suggests disruption of endometrial receptivity, likely due to toxic constituents such as phorbol esters or lectins2. In contrast, the petroleum ether extract showed a milder and potentially reversible antifertility effect.
The vaginal histological alterations, although not directly linked to implantation failure, may further hinder fertility through altered pH, hormonal imbalance, or mucosal immune response. The absence of damage in ovaries and fallopian tubes suggests the mechanism is localized, likely affecting post-ovulatory processes such as endometrial preparation and embryo adhesion11.
Our study on the antifertility and anti-implantation effects of Croton tiglium seed extracts aligns with several recent studies investigating plant-based contraceptives and their effects on reproductive health. The complete inhibition of implantation observed in the ethanol group corresponds with findings from Zhang et al.2 who demonstrated that plant-derived compounds can disrupt uterine receptivity through endometrial toxicity. Similarly, our histopathological observations of uterine damage, including hyperemia, glandular necrosis, and epithelial erosion, echo the work of Alchalabi et al.11 who noted similar uterine changes following the administration of toxic compounds in rats.
|
|

|
In our study, the petroleum ether extract caused milder antifertility effects, a finding that is consistent with David et al.9 who reported dose-dependent toxicity of Croton tiglium extracts, with less severe impacts when less potent solvents were used. The localized toxicity in the uterus and vagina, without ovarian involvement, supports the findings of Solomon et al.10 who suggested that some antifertility agents specifically affect uterine and vaginal tissues, leaving ovarian function unaffected.
Histopathological changes in the vagina, including epithelial hyperplasia and lymphocytic infiltration, indicate potential estrogenic or inflammatory effects, which align with the observations of Abebe et al.8 who also noted such histological changes following exposure to plant-based contraceptives. These changes may affect sperm survival and further contribute to infertility, as suggested by Fadeyi et al.12, who observed similar alterations in vaginal tissue following hormonal treatment.
Additionally, current results were supported by studies investigating the biochemical activity of Croton tiglium seeds. The presence of phorbol esters in C. tiglium is linked to cellular toxicity, which may explain the significant uterine and vaginal changes observed in the ethanol-treated group2. The potential role of phorbol esters in disrupting the uterine environment and preventing implantation has been highlighted in previous research of Chopra et al.3 Sinsinwar et al.4.
The findings of current study contribute to the growing body of evidence suggesting that plant extracts, particularly those containing toxic compounds like phorbol esters, hold promise as contraceptive agents. However, the observed high mortality and toxicity underscore the need for further studies to optimize dosages and evaluate long-term safety profiles before considering therapeutic applications.
CONCLUSION
Results showed that the ethanol extract caused more profound uterine and vaginal histopathological changes than the petroleum ether extract, aligning with its greater antifertility efficacy but also higher toxicity. Mortality in the ethanol-treated group underscores the need for dose optimization and safety profiling before considering therapeutic application. Croton tiglium seeds contain phorbol esters, croton oil, and alkaloids, which are known to activate PKC pathways, induce inflammation, and disrupt endometrial integrity. These effects can alter uterine receptivity by accelerating ovum transit, Disrupting endometrial preparation and Inducing vaginal histological changes.
SIGNIFICANCE STATEMENT
This study provides experimental validation for the traditional contraceptive use of Croton tiglium seeds by demonstrating significant antifertility and anti-implantation effects, particularly with the ethanol extract. The observed disruption of uterine and vaginal histology highlights a potential mechanism of localized reproductive interference without affecting ovarian structure. These findings not only support the ethnomedicinal claims but also emphasize the need for caution due to associated toxicity. The study contributes to the development of plant-derived contraceptives and reinforces the importance of rigorous pharmacological and toxicological evaluation of traditional remedies.
REFERENCES
- Aboulthana, W.M., A.M. Youssef, A.M. El-Feky, N.E.S. Ibrahim, M.M. Seif and A.K. Hassan, 2019. Evaluation of antioxidant efficiency of Croton tiglium L. seeds extracts after incorporating silver nanoparticles. Egypt. J. Chem., 62: 181-200.
- Zhang, T., Z. Liu, X. Sun, Z. Liu and L. Zhang et al., 2022. Botany, traditional uses, phytochemistry, pharmacological and toxicological effects of Croton tiglium Linn.: A comprehensive review. J. Pharm. Pharmacol., 74: 1061-1084.
- Chopra, R., S.L. Nayar and I.C. Chopra, 1956. Glossary of Indian Medicinal Plants. 1st Edn., Council of Scientific & Industrial Research, New Delhi, India, Pages: 329.
- Sinsinwar, S., I. Paramasivam and M.S. Muthuraman, 2016. An overview of the biological and chemical perspectives of Croton tiglium. Der Pharmacia Lett., 8: 324-328.
- Fadeyi, O.J., N.A. Akwu, M. Lekhooa, R. Hayeshi and A.O. Aremu, 2024. Utilisation of medicinal plants for their antifertility activities: A bibliometric analysis of research endeavours from 1968 to 2023. Phytomed. Plus, 4.
- Oludare, G.O. and B.O. Iranloye, 2016. Implantation and pregnancy outcome of Sprague-Dawley rats fed with low and high salt diet. Middle East Fertil. Soc. J., 21: 228-235.
- Zar, J.H., 2010. Biostatistical Analysis. 5th Edn., Prentice-Hall, Upper Saddle River, New Jersey, Englewood, USA., ISBN-13: 9780131008465, Pages: 960.
- Abebe, M.S., K. Asres, Y. Bekuretsion, S. Woldekidan and E. Debebe et al., 2023. Toxic effect of Syzygium guineense ethanolic extract on female reproduction in rats: An evidence from a 10 week repeated-dose toxicity study. Heliyon, 9.
- David, M., Qurat Ul Ain, S. Jahan, M. Ahmad and Q. Shah et al., 2022. Determination of possible contraceptive potential of methanolic leaf extract of Mentha longifolia L. in adult male rats: A biochemical and histological study. Toxicol. Res., 11: 951-961.
- Solomon, T., Z. Largesse, A. Mekbeb, M. Eyasu and D. Asfaw, 2010. Effect of Rumex steudelii methanolic root extract on ovarian folliculogenesis and uterine histology in female albino rats. Afr. Health Sci., 10: 353-361.
- Alchalabi, A.S.H., H. Rahim, E. Aklilu, I.I. Al-Sultan, A.R. Aziz et al., 2016. Histopathological changes associated with oxidative stress induced by electromagnetic waves in rats' ovarian and uterine tissues. Asian Pac. J. Reprod., 5: 301-310.
- Fadeyi, O.J., M. Lekhooa, M.A. Moroole, C. Bester, A.O. Aremu and R. Hayeshi, 2024. Chemical composition and antifertility effect of a South African herbal mixture in female Sprague-Dawley rats. S. Afr. J. Bot., 170: 394-400.
How to Cite this paper?
APA-7 Style
EL-Kamali,
H.H., Mahjoub,
S.E., Omran,
A.M. (2025). Antifertility and Anti-Implantation Effects of Croton tiglium Seed Extracts in Female Albino Rats. Trends in Biological Sciences, 1(2), 200-208. https://doi.org/10.21124/tbs.2025.200.208
ACS Style
EL-Kamali,
H.H.; Mahjoub,
S.E.; Omran,
A.M. Antifertility and Anti-Implantation Effects of Croton tiglium Seed Extracts in Female Albino Rats. Trends Biol. Sci 2025, 1, 200-208. https://doi.org/10.21124/tbs.2025.200.208
AMA Style
EL-Kamali
HH, Mahjoub
SE, Omran
AM. Antifertility and Anti-Implantation Effects of Croton tiglium Seed Extracts in Female Albino Rats. Trends in Biological Sciences. 2025; 1(2): 200-208. https://doi.org/10.21124/tbs.2025.200.208
Chicago/Turabian Style
EL-Kamali, Hatil, Hashim Ahmed, Sana EL-Tayeb Mahjoub, and Awatif M.E. Omran.
2025. "Antifertility and Anti-Implantation Effects of Croton tiglium Seed Extracts in Female Albino Rats" Trends in Biological Sciences 1, no. 2: 200-208. https://doi.org/10.21124/tbs.2025.200.208

This work is licensed under a Creative Commons Attribution 4.0 International License.




